jamesp
Cave Dweller 
Member since October 2012
Posts: 36,154
|
Post by jamesp on Jan 30, 2023 8:35:27 GMT -5
Where would RTH be without silicates Dave ? Or for that matter where would earth be without it. Maybe earth will be reformed some day to have only perfect gem grade silicon dioxide compound minerals with no fractures. Like perfectly clear AAA 10 pound ruby/aquamarine/emerald crystals and millions of varieties of perfect agate nodules. "As mentioned in the case of silicate, silicon is also a prominent element found in the Earth’s crust. It makes up some 28% of the crust, and can be found in a wide variety of minerals and elemental compounds, usually in conjunction with oxygen. Silicon dioxide is one of the most common of these compounds, and is composed of silicon and oxygen. Silicon dioxide is the main component of many types of hard crystalline rocks such as quartz, amethyst, opal and rock crystal. Silicon Dioxide is also what most sand is made out of, and a large part of the reason it is so commonly found in the earth’s crust. Sand is mostly made up of silicon based minerals and rocks. Silicon is also used in a variety of human made products such as most electronics, and microchips as well as glass products and bricks." 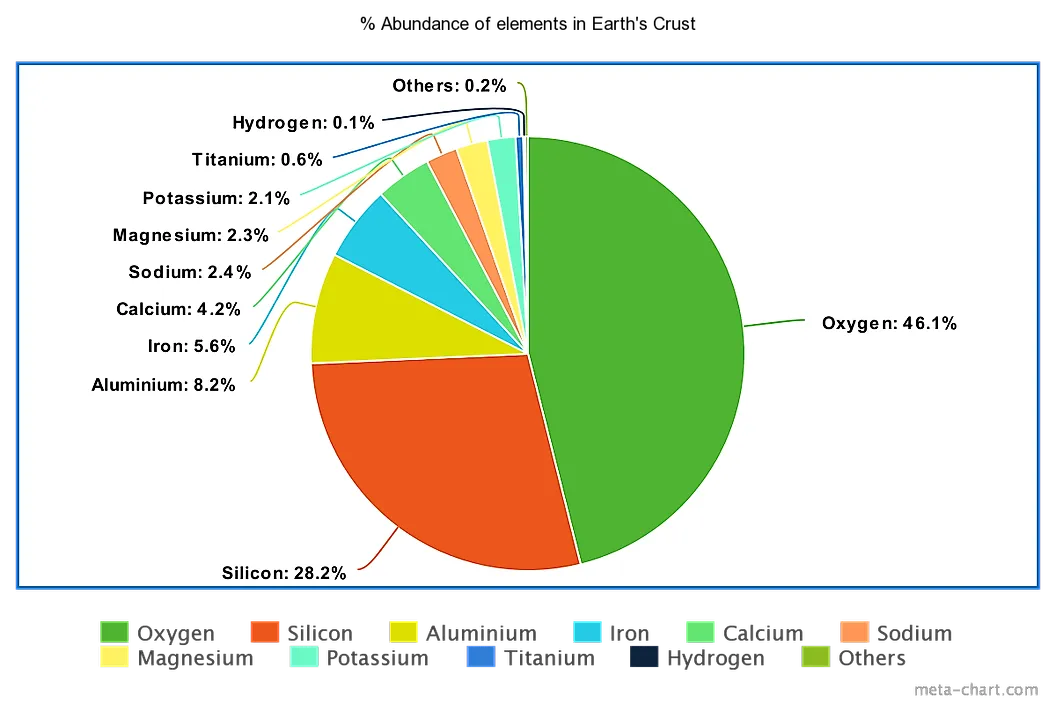 |
|
|
|
Post by 1dave on Jan 30, 2023 12:31:05 GMT -5
Ah jamesp Who knows what evil lies at the heart of ROCKS? The Belousov-Zhabotinsky (BZ) reaction is a family of oscillating chemical reactions. During these reactions, transition-metal ions catalyze oxidation of various, usually organic, reductants by bromic acid in acidic water solution. Most BZ reactions are homogeneous. The BZ reaction makes it possible to observe development of complex patterns in time and space by naked eye on a very convenient human time scale of dozens of seconds and space scale of several millimeters. The BZ reaction can generate up to several thousand oscillatory cycles in a closed system, which permits studying chemical waves and patterns without constant replenishment of reactants (Field and Burger, 1985; Epstein and Showalter, 1996; Epstein and Pojman, 1998; Taylor, 2002) |
|
jamesp
Cave Dweller 
Member since October 2012
Posts: 36,154
|
Post by jamesp on Jan 31, 2023 11:35:29 GMT -5
That video appears to display "changing rock fingerprints" 1dave. Speaking of the silica subject, compounds of silica is the most common 'rock' or solid on earth but has more personalities than Carter's had pills. Remove all silica and us rock lovers would be in trouble. There would be no agates, most gemstones, quartz, granites etc etc. The more one studies silica compounds the more confused they get, my experience anyway. That is why there are Daves on earth(to comprehend such)! So is nature. The most common is often the most complicated. The most obvious is often the most deceptive. What feels the best can cause the most harm. What looks good is often the most evil. What seems old is new or new is old. One assumption often leads to large error. Two assumptions often leads to extreme error. Multiple assumptions often leads to infinite error. |
|
|
|
Post by 1dave on Jan 31, 2023 12:56:23 GMT -5
|
|
jamesp
Cave Dweller 
Member since October 2012
Posts: 36,154
|
Post by jamesp on Jan 31, 2023 15:39:16 GMT -5
Ha, Georgia redneck philosophy Dave. |
|
|
|
Post by 1dave on Feb 1, 2023 0:03:52 GMT -5
jamesp I'm trying to understand a new concept: Hydrophobic Rocks!  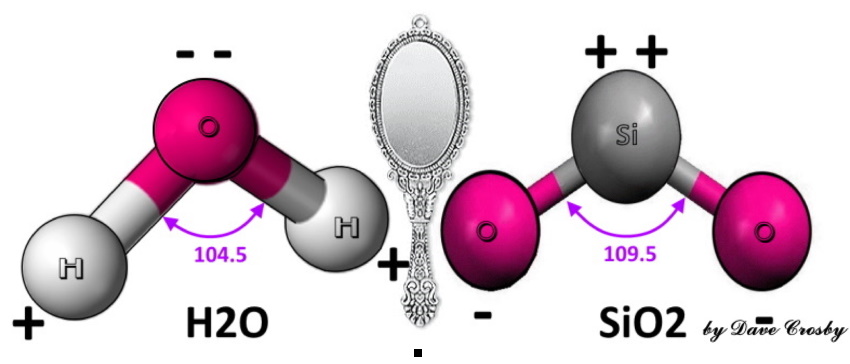 the pH of the hydrolyzing compound dictates the degree to which it is hydrophilic or hydrophobic. Acidic pH produces hydrophilic siloxanes. In a more alkaline environment, the siloxane will tend to be more hydrophobic. Once the Si-O bonding type is formed (either hydrophobic or hydrophilic), later acidification or alkaline change does not alter the nature of the Si-O bonding. The dissolved SiO exhibits a significant vapor pressure, and when cooled will nucleate to form sub-micronic fibroids which remain hydrophobic. with hydrogen Si-OH. As simple monomers and dimers, these compounds are normally hydrophilic and referred to as siloxanes or silanols (the silicon analogs to ketones and alcohols). In polymeric form, these cross-linked silicon-oxygen bonded compounds are generally hydrophobic and referred to as silicones. 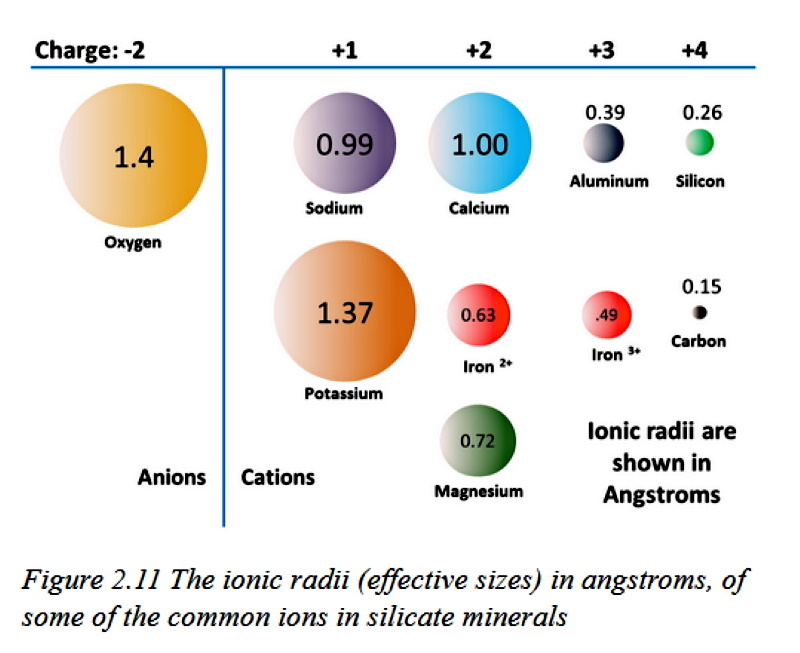 It needs a lot of new drawings to make heads or tails of. icon_think
|
|
jamesp
Cave Dweller 
Member since October 2012
Posts: 36,154
|
Post by jamesp on Feb 1, 2023 7:23:46 GMT -5
jamesp I'm trying to understand a new concept: Hydrophobic Rocks!   the pH of the hydrolyzing compound dictates the degree to which it is hydrophilic or hydrophobic. Acidic pH produces hydrophilic siloxanes. In a more alkaline environment, the siloxane will tend to be more hydrophobic. Once the Si-O bonding type is formed (either hydrophobic or hydrophilic), later acidification or alkaline change does not alter the nature of the Si-O bonding. The dissolved SiO exhibits a significant vapor pressure, and when cooled will nucleate to form sub-micronic fibroids which remain hydrophobic. with hydrogen Si-OH. As simple monomers and dimers, these compounds are normally hydrophilic and referred to as siloxanes or silanols (the silicon analogs to ketones and alcohols). In polymeric form, these cross-linked silicon-oxygen bonded compounds are generally hydrophobic and referred to as silicones.  It needs a lot of new drawings to make heads or tails of. icon_think This might be a related subject from a more basic standpoint Dave: Hard or alkaline water is slow to remove soap film in shower. Leaves skin soapy slick. Acid water removes soap easily. Leaves skin squeaky clean. Both have benefits and drawbacks for skin health. Or high acid jungle runoff water cuts/dissolves grooves in solid limestone river bluff walls as it flows over them. Silicified fossils in and around said limestone that are normally white/pale will pick up/absorb color from the metal salts in the vicinity of an ancient acid water runoff at such low land runoff arrangements. Florida coral is a great example of this phenom. Salt water has a whole different effect on colors of silicifications since it dissolves metals so readily and has a plethora of other chemicals. "Hard water is water that has high mineral content (in contrast with "soft water"). Hard water is formed when water percolates through deposits of limestone, chalk or gypsum, which are largely made up of calcium and magnesium carbonates, bicarbonates and sulfates. Hard drinking water may have moderate health benefits." Many jungles/tropical forests grow on top of limestone bedrock, and the forest generates acids due to organic matter rotting. The chemical reaction of the acidic water leaching down to the alkaline limestone bedrock dissolves powerful fertilizers feeding the massive forest. A fine organic compost fertilizer allowing massive plant production. |
|
|
|
Post by 1dave on Feb 1, 2023 8:50:38 GMT -5
Thanks jamesp, I'm so glad to have you as a friend to bounce ideas of of! I LIKE that perspective. This morning I'm thinking of a row of silicon motels on a busy highway with H- and OH+ water molecules racing by. Each motel has 4 vacancies.  If it fills up with OH+ customers, the owner happily watches the cash flow in and is homophobically satisfied. If it is on an acidic street, only H- customers drive by and it is homophilic, in and out customers. Does that work? |
|
jamesp
Cave Dweller 
Member since October 2012
Posts: 36,154
|
Post by jamesp on Feb 1, 2023 15:18:20 GMT -5
If on an acidic street it would depend on the type of acid customers(LSD users for instance  ). In either case do not mention to customers you are looking for homophobic customers. Probably a bad idea to refer to them as homophilic customers too. Hemophiliac customers should not be a problem but may raise curiosity. More corny perspectives from jamesp. Always enjoy your perspectives especially if they can be grasped with my common mind. |
|