Post by 1dave on Jan 13, 2016 11:23:48 GMT -5
An Extension of Geology for Rockhounds.
We stand on the shoulders of Giants, most of them we will never know.
Of the billions of people that have lived on earth only a very few have contributed to what we know.
Much of what was learned has been lost, re-discovered, and then lost again.
We would know nothing if a few ancient ideas hadn’t survived for us to build on.

The ancients only knew of four “elements” - Earth, air, fire, and water.
They slowly became aware of 9 real elements:
Carbon, Sulfur, and the 7 metals - Iron, Copper, Silver, Gold, Mercury, Tin and Lead.
The discovery of tin initiated the Bronze Age in different parts of the globe.
-Near East (c. 3300–1200 BC)
-Europe (c. 3200–600 BC)
-South Asia (c. 3000– 1200 BC)
-China (c. 2000–700 BC)
465BC - #1. Leucippus (there is doubt that he existed) In Greece came up with an atomic theory:

400BC -#2. His student, Democritus (Abdera, Greece, 460BC - 370BC), expanded it.
His version of atomic theory stated that atoms were minuscule quantities of matter that cannot be destroyed, differ in size, shape and temperature, are always moving, and are invisible. He believed that there are an infinite number of atoms.
The Dark Ages

1662 - #3 Robert Boyle (25 January 1627 – 31 December 1691) Boyle's Law, published in 1662, states that:
By 1700, 6 more elements (all metals) had been discovered:
Zinc (converting copper into brass), Phosphorus, Arsenic, Antimony, Bismuth, and Platinum.
Then came the golden years of discovery, 1735-1843 - 42 new elements!
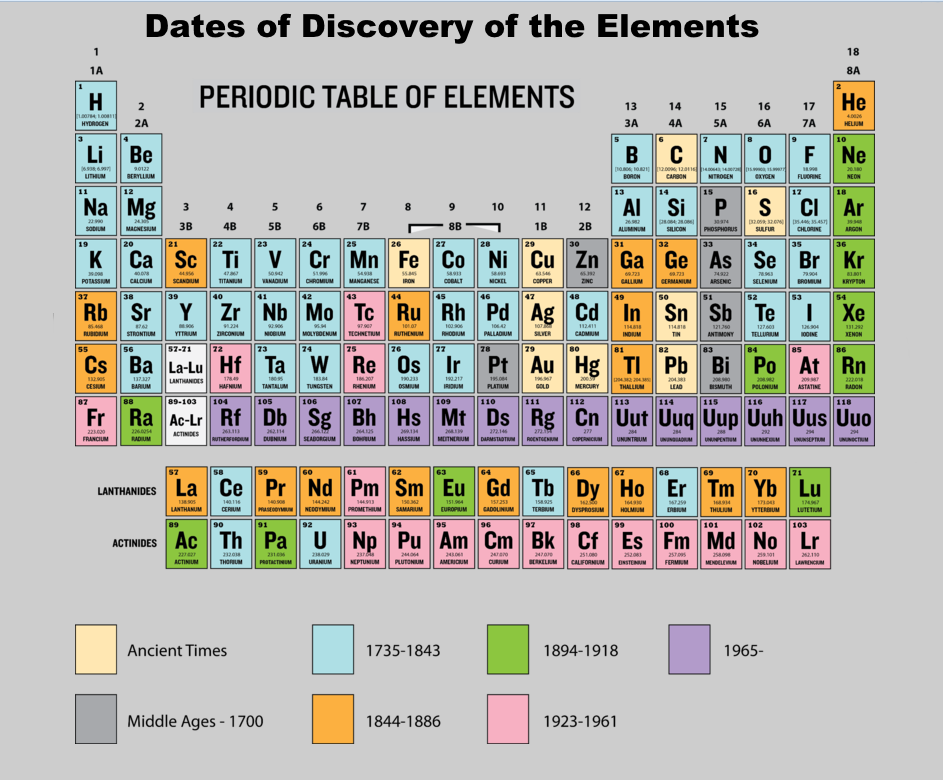
Chart 1.

1785 - #4 James Hutton (14 June 1726 NS – 26 March 1797) “The father of Modern Geology.”
Through observation and carefully reasoned arguments, he came to believe that the Earth was perpetually being formed, and recognized that the history of the Earth could be determined by understanding how the processes of vulcanism, erosion and sedimentation work in the present day.

1787 - #5 Jacques Charles (November 12, 1746 – April 7, 1823) The Father of the Hydrogen Balloon, deduced Charles' Law, or the law of volumes. It states that

1803 - #6. John Dalton, led by his search for an explanation of his “law of multiple proportions” to the idea that chemical combination consists in the interaction of atoms of definite and characteristic weight, the idea of atoms arose in his mind as a purely physical concept, forced upon him by study of the physical properties of the atmosphere and other gases. The first published indications of this idea are to be found at the end of his paper on the absorption of gases, which was read on 21 October 1803, though not published until 1805. Here he says:
Why does not water admit its bulk of every kind of gas alike? This question I have duly considered, and though I am not able to satisfy myself completely I am nearly persuaded that the circumstance depends on the weight and number of the ultimate particles of the several gases.
The main points of Dalton's atomic theory were:
This was merely an assumption, derived from faith in the simplicity of nature. No evidence was then available to scientists to deduce how many atoms of each element combine to form compound molecules. But this or some other such rule was absolutely necessary to any incipient theory, since one needed an assumed molecular formula in order to calculate relative atomic weights. In any case, Dalton's "rule of greatest simplicity" caused him to assume that the formula for water was OH and ammonia was NH, quite different from our modern understanding (H2O, NH3).

1809 - #7 Joseph Louis Gay-Lussac (1778–1850) formulated Gay-Lussac's Law, or the Pressure Law. It states that,

1811 - #8 Amedeo Avogadro (9 August 1776 – 9 July 1856) Avogadro's law states that,
For a given mass of an ideal gas, the volume and amount (moles) of the gas are directly proportional if the temperature and pressure are constant.
Combining ALL the gas laws, Avogadro, Boyle, Charles, and Gay-Lussac brought the invisible elements into view.

1829, - #9. Johann Wolfgang Dobereiner (13 December 1780 – 24 March 1849), a German scientist, was the first to classify elements into groups based on John Dalton's assertion that the atoms of an element have a characteristic mass.
He grouped the elements with similar chemical properties into clusters of three called 'Triads'. The distinctive feature of a triad was the atomic mass of the middle element. When elements were arranged in order of their increasing atomic mass, the atomic mass of the middle element was approximately the arithmetic mean of the other two elements of the triad.
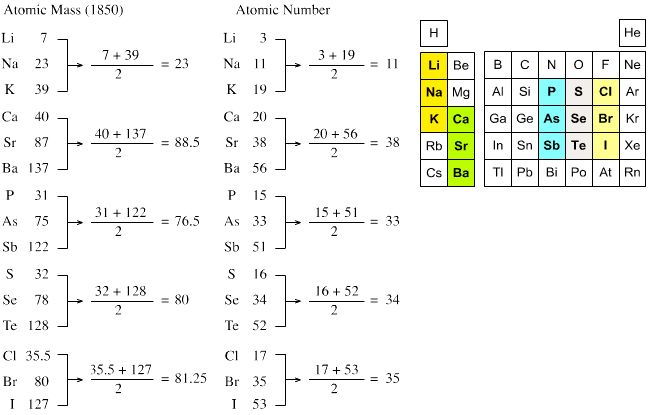
Chart 2.

1830 - #10 Charles Lyell published “Principles of Geology.”

1837 - #11 James Dwight Dana (February 12, 1813 – April 14, 1895), a friend of #13 - George Jarvis Brush, was an American geologist, mineralogist, volcanologist, and zoologist. He made pioneering studies of mountain-building, volcanic activity, and the origin and structure of continents and oceans around the world. He gathered and published the most comprehensive information for identifying the elements and the minerals they formed ever published.
Dana's best known books were his


System of Mineralogy (1837)
Manual of Mineralogy (1848), and his Manual of Geology (1863).
A bibliographical list of his writings shows 214 titles of books and papers, beginning in 1835 with a paper on the conditions of Vesuvius in 1834. His reports on Zoophytes, on the Geology of the Pacific Area, and on Crustacea, summarizing his work on the Wilkes Expedition, appeared from 1846 onward. Other works included Manual of Mineralogy (1848), afterwards entitled Manual of Mineralogy and Lithology (ed. 4, 1887); and Corals and Coral Islands] (1872; revised ed. 1890). In 1887, Dana revisited the Hawaiian Islands, and the results of his further investigations were published in a quarto volume entitled Characteristics of Volcanoes (1890).
Dana's System of Mineralogy has also been revised, the 6th edition (1892)[6] being edited by his son, #17 - Edward Salisbury Dana. A 7th edition was published in 1944, and the 8th edition was published in 1997 under the title Dana's New Mineralogy, edited by R. V. Gaines et al.
Between 1856 and 1857, Dana published a number of manuscripts in an effort to reconcile scientific findings with the Bible. Among these, he wrote Science and the Bible: A Review of the Six Days of Creation (1856), and Creation, Or, The Biblical Cosmogony in the Light of Modern Science (1885).

1869 - #12. Dmitri Ivanovich Mendeleev (1834 – 1907).
Mendeleev and Moseley are credited with being most responsible for the modern periodic law, which states that
In his time, chemistry was a patchwork of guesses, observations and discoveries.
Mendeleev was certain that better, more fundamental principles could be found; this was his mindset when, in 1869, he began writing a second volume of his book The Principles of Chemistry.
At the heart of chemistry were its elements. What, wondered Mendeleev, could they reveal to him if he could find some way of organizing them logically?
He wrote the names of the 65 known elements on cards – much like playing cards – one element on each card. He then wrote the fundamental properties of every element on its own card, including atomic weight. He saw that atomic weight was important in some way – the behavior of the elements seemed to repeat as their atomic weights increased – but he could not see the pattern.
Convinced that he was close to discovering something significant, Mendeleev moved the cards about for hour after hour until finally he fell asleep at his desk.
When he awoke, he found that his subconscious mind had done his work for him! He now knew the pattern the elements followed. He later wrote:
“In a dream I saw a table where all the elements fell into place as required. Awakening, I immediately wrote it down on a piece of paper.”
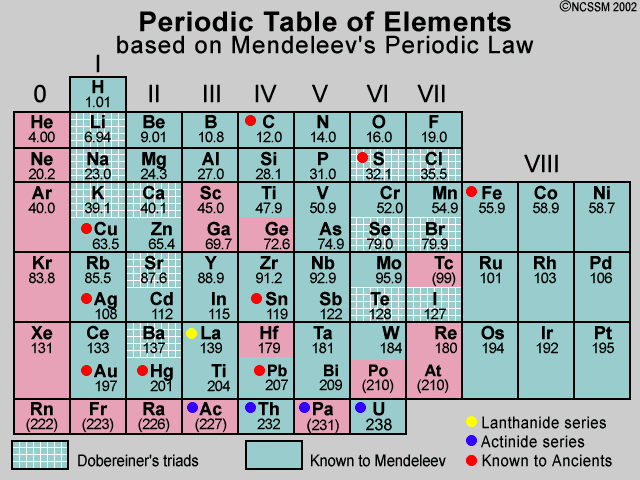
Chart 3.
The result is the periodic table as we know it today (Figure below).
We now know that each new horizontal row of the periodic table corresponds to the beginning of a new principal energy level being filled with electrons. Elements with similar chemical properties appear at regular intervals, within the vertical columns called groups.
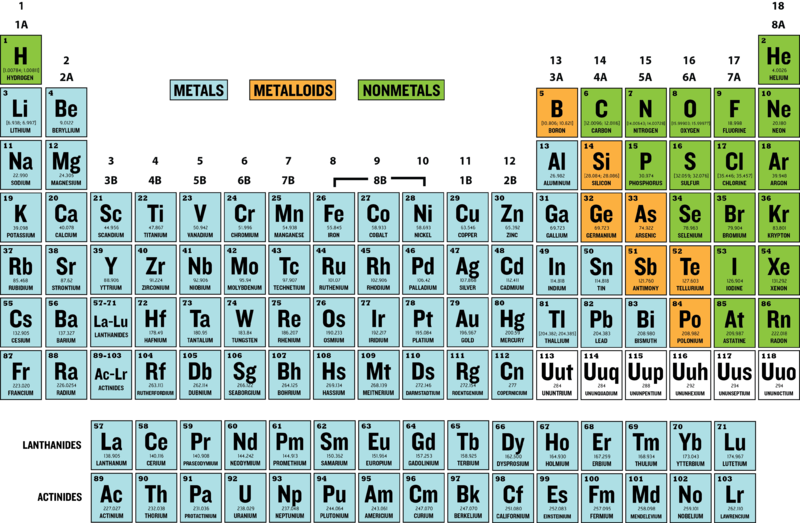
Chart 4.
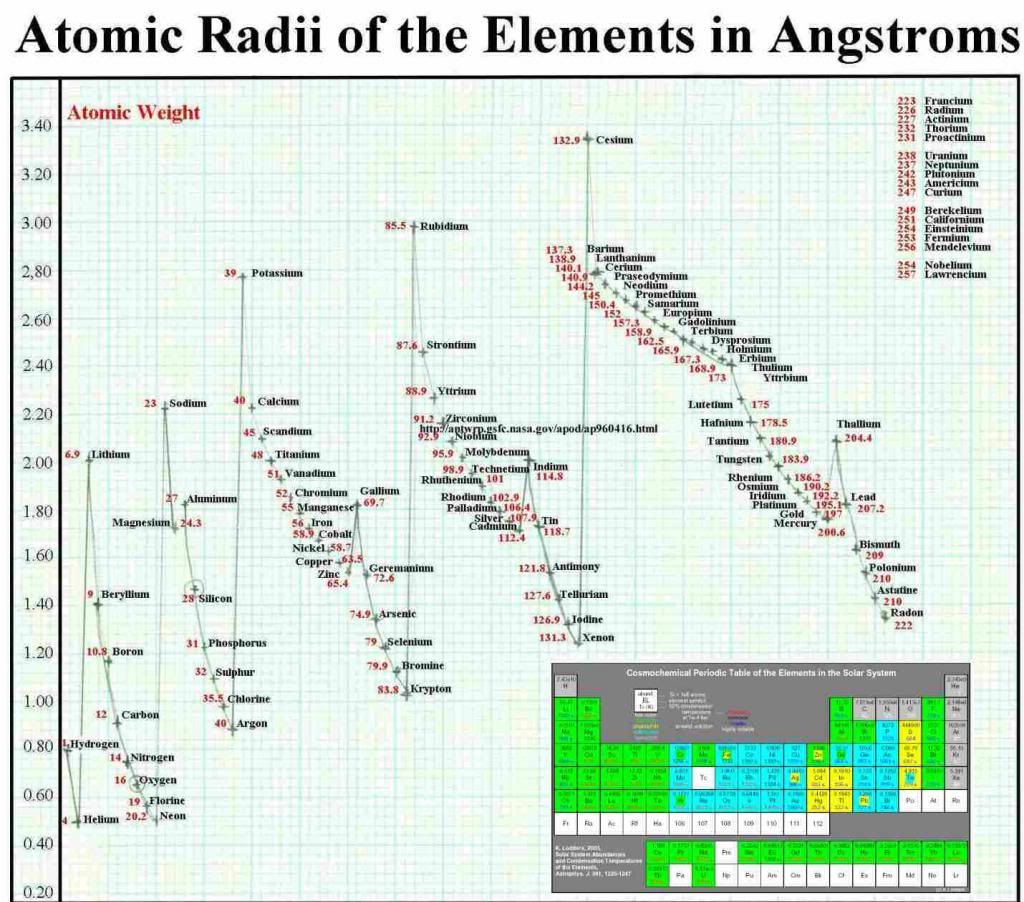
Chart 5.


1875 - #13 George Jarvis Brush (1831–1912) was an American mineralogist born in Brooklyn, New York. He began his studies at Yale in 1848 with courses from Benjamin Silliman, Jr. and John Pitkin Norton on practical chemistry and agriculture. He also studied chemistry, metallurgy and mineralogy. From 1852 to 1855, Brush worked and studied at the University of Virginia and in Munich and Freiberg. He returned to Sheffield in 1855 to join the faculty as professor of Metallurgy and later of Mineralogy. Brush had begun acquiring an extensive research collection of minerals. He was appointed the first curator of the Peabody Museum of Natural History's mineral collection. He published extensively in the American Journal of Science and other journals.
He also published a Manual of Determinative Mineralogy (1875; fifteenth edition, 1899).

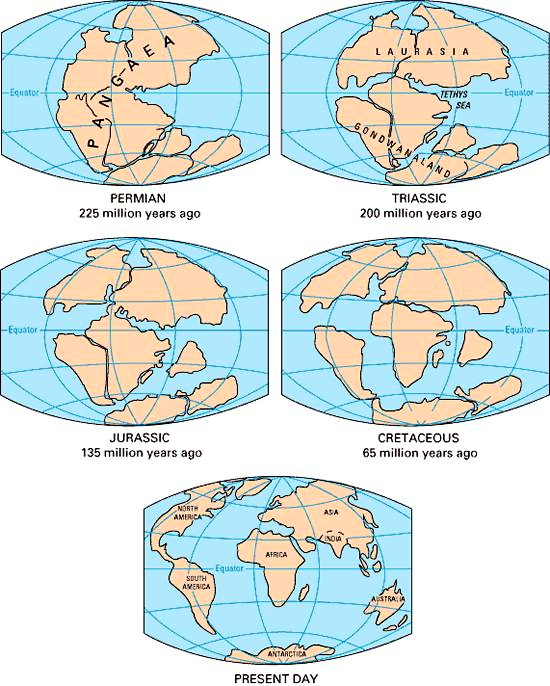
1912 - #14 Alfred Lothar Wegener (1 November 1880 – November 1930) suggested The theory of Continental drift, but the idea wasn’t accepted until circa 1955.
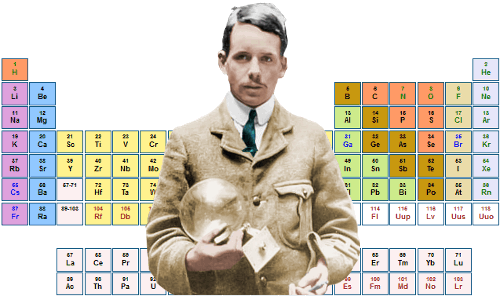
1913 - #15 Henry Gwyn Jeffreys Moseley (23 November 1887 – 10 August 1915)Perfected the Periodic table.
Probably would have become one of our most esteemed scientists had he not been killed at the tender age of 27 in the First World War by a Muslim sniper bullet.
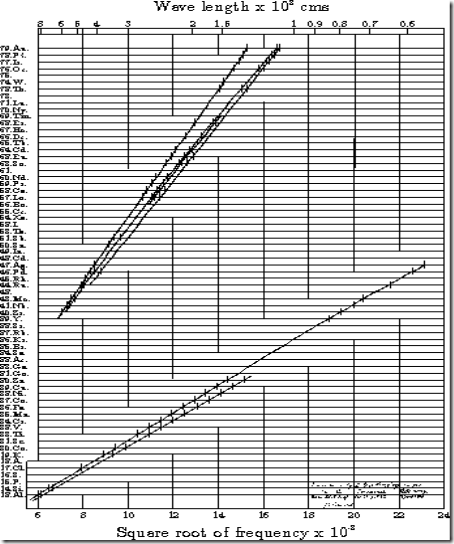
In 1913 - Moseley observed and measured the X-ray spectra of various chemical elements (mostly metals) that were found by the method of diffraction through crystals. This was a pioneering use of the method of X-ray spectroscopy in physics, using Bragg's diffraction law to determine the X-ray wavelengths. Moseley discovered a systematic mathematical relationship between the wavelengths of the X-rays produced and the atomic numbers of the metals that were used as the targets in X-ray tubes. This has become known as Moseley's law.
Moseley's Law (spectroscopy)
The law that
Before Moseley's discovery, the atomic numbers (or elemental number) of an element had been thought of as a semi-arbitrary sequential number, based on the sequence of atomic masses, but modified somewhat where chemists found this modification to be desirable, such as by the Russian chemist, Dmitri Ivanovich Mendeleev.
In his invention of the Periodic Table of the Elements, Mendeleev had interchanged the orders of a few pairs of elements in order to put them in more appropriate places in this table of the elements. For example, the metals cobalt and nickel had been assigned the atomic numbers 27 and 28, respectively, based on their known chemical and physical properties, even though they have nearly the same atomic masses. In fact, the atomic mass of cobalt is slightly larger than that of nickel, which would have placed them in backwards order if they had been placed in the Periodic Table blindly according to atomic mass.
Moseley's experiments in X-ray spectroscopy showed directly from their physics that cobalt and nickel have the different atomic numbers, 27 and 28, and that they are placed in the Periodic Table correctly by Moseley's objective measurements of their atomic numbers. Hence, Moseley's discovery demonstrated that the atomic numbers of elements are not just rather arbitrary numbers based on chemistry and the intuition of chemists, but rather, they have a firm experimental basis from the physics of their X-ray spectra.
In addition, Moseley showed that there were gaps in the atomic number sequence at numbers 43, 61, 72, and 75. These spaces are now known, respectively, to be the places of the radioactive synthetic elements technetium and promethium, and also the last two quite rare naturally occurring stable elements hafnium (discovered 1923) and rhenium (discovered 1925). Nothing about these four elements was known of in Moseley's lifetime, not even their very existence. Based on the intuition of a very experienced chemist, Dmitri Mendeleev had predicted the existence of a missing element in the Periodic Table, which was later found to be filled by technetium, and Bohuslav Brauner had predicted the existence of another missing element in this Table, which was later found to be filled by promethium. Henry Moseley's experiments confirmed these predictions, by showing exactly what the missing atomic numbers were, 43 and 61. In addition, Moseley predicted the two more undiscovered elements, those with the atomic numbers 72 and 75, and gave very strong evidence that there were no other gaps in the Periodic Table between the elements aluminum (atomic number 13) and gold (atomic number 79).
This latter question about the possibility of more undiscovered ("missing") elements had been a standing problem among the chemists of the world, particularly given the existence of the large family of the lanthanide series of rare earth elements. Moseley was able to demonstrate that these lanthanide elements, i.e. lanthanum through lutetium, must have exactly 15 members – no more and no less. The number of elements in the lanthanides had been a question that was very far from being settled by the chemists of the early 20th Century. They could not yet produce pure samples of all the rare-earth elements, even in the form of their salts, and in some cases they were unable to distinguish between mixtures of two very similar (adjacent) rare-earth elements from the nearby pure metals in the Periodic Table. For example, there was a so-called "element" that was even given the chemical name of "didymium". "Didymium" was found some years later to be simply a mixture of two genuine rare-earth elements, and these were given the names neodymium and praseodymium, meaning "new twin" and "green twin". Also, the method of separating the rare-earth elements by the method of ion exchange had not been invented yet in Moseley's time.
Moseley's method in early X-ray spectroscopy was able to sort out the above chemical problems promptly, some of which had occupied chemists for a number of years. Moseley also predicted the existence of element 61, a lanthanide whose existence was previously unsuspected. Quite a few years later, this element 61 was created artificially in nuclear reactors and was named promethium.[12]
Isaac Asimov wrote, Because of Moseley's death in World War I, and after much lobbying by Ernest Rutherford,[15] the British government instituted a policy of no longer allowing its prominent and promising scientists to enlist for combat duty in the armed forces of the Crown.[3]
Isaac Asimov also speculated that, in the event that he had not been killed while in the service of the British Empire, Moseley might very well have been awarded the 1916 Nobel Prize in Physics, which, along with the prize for chemistry, was not awarded to anyone that year.

1928 - #16 Norman Levi Bowen was born in Kingston, Ontario, Canada June 21, 1887 and died on September 11, 1956. Bowen "revolutionized experimental petrology and our understanding of mineral crystallization". Beginning geology students are familiar with Bowen's reaction series depicting how different minerals crystallize under varying pressures and temperatures."[3]
Bowen conducted experimental research at the Geophysical Laboratory, Carnegie Institution for Science of Washington from 1912 to 1937. He published The Evolution of the Igneous Rocks in 1928. This book set the stage for a geochemical and geophysical foundation for the study of rocks and minerals. This book became the petrology handbook.




1944 - #17 Edward Salisbury Dana (November 16, 1849 – June 16, 1935), the son of #11 - James Dana, was an American mineralogist and physicist. He made important contributions to the study of minerals, especially in the field of crystallography.
E. S. Dana was born in New Haven, Connecticut, the son of the geologist and mineralogist James Dwight Dana.[1] He graduated from Yale College in 1870, where he had been a member of Scroll and Key, and then after two years with George J. Brush at the Sheffield Scientific School, spent another two years studying in Heidelberg and Vienna, specializing in crystal optics and crystallography. He then returned to Yale to take his M.A. and Ph.D. degrees. He was a member of the Connecticut Academy of Arts and Sciences.
He was appointed assistant professor of natural philosophy and astronomy at Yale in 1879 and then became professor of physics.[1][2] His research and publishing was mainly in the field of mineralogy.
Dana became an editor of the American Journal of Science in 1875 and continued to direct it until 1926. In 1884 was elected a member of the National Academy of Sciences. In 1885 he was made a trustee of the Peabody Museum of Yale.[2] He was an elected member of scientific societies in Austria, Mexico, Russia, England, Scotland, and across the United States.

From Amazon.com:
We stand on the shoulders of Giants, most of them we will never know.
Of the billions of people that have lived on earth only a very few have contributed to what we know.
Much of what was learned has been lost, re-discovered, and then lost again.
We would know nothing if a few ancient ideas hadn’t survived for us to build on.

The ancients only knew of four “elements” - Earth, air, fire, and water.
They slowly became aware of 9 real elements:
Carbon, Sulfur, and the 7 metals - Iron, Copper, Silver, Gold, Mercury, Tin and Lead.
The discovery of tin initiated the Bronze Age in different parts of the globe.

-Near East (c. 3300–1200 BC)
-Europe (c. 3200–600 BC)
-South Asia (c. 3000– 1200 BC)
-China (c. 2000–700 BC)
465BC - #1. Leucippus (there is doubt that he existed) In Greece came up with an atomic theory:
“The universe is composed of two elements - the atoms and the void in which they exist and move.”

400BC -#2. His student, Democritus (Abdera, Greece, 460BC - 370BC), expanded it.
His version of atomic theory stated that atoms were minuscule quantities of matter that cannot be destroyed, differ in size, shape and temperature, are always moving, and are invisible. He believed that there are an infinite number of atoms.
Democritus' atomic theory:
1.All matter consists of invisible particles called atoms.
2. Atoms are indestructible.
3. Atoms are solid but invisible.
4. Atoms are homogenous.
5. Atoms differ in size, shape, mass, position, and arrangement.
-Solids are made of small, pointy atoms.
-Liquids are made of large, round atoms.
-Oils are made of very fine, small atoms that can easily slip past each other.
1.All matter consists of invisible particles called atoms.
2. Atoms are indestructible.
3. Atoms are solid but invisible.
4. Atoms are homogenous.
5. Atoms differ in size, shape, mass, position, and arrangement.
-Solids are made of small, pointy atoms.
-Liquids are made of large, round atoms.
-Oils are made of very fine, small atoms that can easily slip past each other.
The Dark Ages

1662 - #3 Robert Boyle (25 January 1627 – 31 December 1691) Boyle's Law, published in 1662, states that:
at constant temperature, the product of the pressure and volume of a given mass of an ideal gas in a closed system is always constant.
By 1700, 6 more elements (all metals) had been discovered:
Zinc (converting copper into brass), Phosphorus, Arsenic, Antimony, Bismuth, and Platinum.
Then came the golden years of discovery, 1735-1843 - 42 new elements!

Chart 1.
1785 - #4 James Hutton (14 June 1726 NS – 26 March 1797) “The father of Modern Geology.”
Through observation and carefully reasoned arguments, he came to believe that the Earth was perpetually being formed, and recognized that the history of the Earth could be determined by understanding how the processes of vulcanism, erosion and sedimentation work in the present day.
Hutton originated the theory of uniformitarianism—a fundamental principle of geology which explains the features of the Earth's crust by means of natural processes over geologic time.

1787 - #5 Jacques Charles (November 12, 1746 – April 7, 1823) The Father of the Hydrogen Balloon, deduced Charles' Law, or the law of volumes. It states that
for a given mass of an ideal gas in a closed system at constant pressure, the volume is directly proportional to its absolute temperature.

1803 - #6. John Dalton, led by his search for an explanation of his “law of multiple proportions” to the idea that chemical combination consists in the interaction of atoms of definite and characteristic weight, the idea of atoms arose in his mind as a purely physical concept, forced upon him by study of the physical properties of the atmosphere and other gases. The first published indications of this idea are to be found at the end of his paper on the absorption of gases, which was read on 21 October 1803, though not published until 1805. Here he says:
Why does not water admit its bulk of every kind of gas alike? This question I have duly considered, and though I am not able to satisfy myself completely I am nearly persuaded that the circumstance depends on the weight and number of the ultimate particles of the several gases.
The main points of Dalton's atomic theory were:
1. Elements are made of extremely small particles called atoms.
2. Atoms of a given element are identical in size, mass, and other properties; atoms of different elements differ in size, mass, and other properties.
3. Atoms cannot be subdivided, created, or destroyed.
4. Atoms of different elements combine in simple whole-number ratios to form chemical compounds.
5. In chemical reactions, atoms are combined, separated, or rearranged.
6. Dalton proposed an additional "rule of greatest simplicity" that created controversy, since it could not be independently confirmed.
When atoms combine in only one ratio, "..it must be presumed to be a binary one, unless some cause appear to the contrary".
2. Atoms of a given element are identical in size, mass, and other properties; atoms of different elements differ in size, mass, and other properties.
3. Atoms cannot be subdivided, created, or destroyed.
4. Atoms of different elements combine in simple whole-number ratios to form chemical compounds.
5. In chemical reactions, atoms are combined, separated, or rearranged.
6. Dalton proposed an additional "rule of greatest simplicity" that created controversy, since it could not be independently confirmed.
When atoms combine in only one ratio, "..it must be presumed to be a binary one, unless some cause appear to the contrary".
This was merely an assumption, derived from faith in the simplicity of nature. No evidence was then available to scientists to deduce how many atoms of each element combine to form compound molecules. But this or some other such rule was absolutely necessary to any incipient theory, since one needed an assumed molecular formula in order to calculate relative atomic weights. In any case, Dalton's "rule of greatest simplicity" caused him to assume that the formula for water was OH and ammonia was NH, quite different from our modern understanding (H2O, NH3).

1809 - #7 Joseph Louis Gay-Lussac (1778–1850) formulated Gay-Lussac's Law, or the Pressure Law. It states that,
for a given mass and constant volume of an ideal gas, the pressure exerted on the sides of its container is directly proportional to its absolute temperature.

1811 - #8 Amedeo Avogadro (9 August 1776 – 9 July 1856) Avogadro's law states that,
"equal volumes of all gases, at the same temperature and pressure, have the same number of molecules".
For a given mass of an ideal gas, the volume and amount (moles) of the gas are directly proportional if the temperature and pressure are constant.
Combining ALL the gas laws, Avogadro, Boyle, Charles, and Gay-Lussac brought the invisible elements into view.
The most significant consequence of Avogadro's law is that the ideal gas constant has the same value for all gases. This led to
the Ideal gas law : pV=nRT
where the letters denote pressure, volume, amount (in moles), ideal gas constant, and temperature of the gas, respectively.
The gas constant value is 8.3144598(48) J mol-1 K-1
The two digits in parentheses are the uncertainty (standard deviation) in the last two digits of the value.
the Ideal gas law : pV=nRT
where the letters denote pressure, volume, amount (in moles), ideal gas constant, and temperature of the gas, respectively.
The gas constant value is 8.3144598(48) J mol-1 K-1
The two digits in parentheses are the uncertainty (standard deviation) in the last two digits of the value.

1829, - #9. Johann Wolfgang Dobereiner (13 December 1780 – 24 March 1849), a German scientist, was the first to classify elements into groups based on John Dalton's assertion that the atoms of an element have a characteristic mass.
He grouped the elements with similar chemical properties into clusters of three called 'Triads'. The distinctive feature of a triad was the atomic mass of the middle element. When elements were arranged in order of their increasing atomic mass, the atomic mass of the middle element was approximately the arithmetic mean of the other two elements of the triad.

Chart 2.

1830 - #10 Charles Lyell published “Principles of Geology.”

1837 - #11 James Dwight Dana (February 12, 1813 – April 14, 1895), a friend of #13 - George Jarvis Brush, was an American geologist, mineralogist, volcanologist, and zoologist. He made pioneering studies of mountain-building, volcanic activity, and the origin and structure of continents and oceans around the world. He gathered and published the most comprehensive information for identifying the elements and the minerals they formed ever published.
Dana's best known books were his


System of Mineralogy (1837)
Manual of Mineralogy (1848), and his Manual of Geology (1863).
A bibliographical list of his writings shows 214 titles of books and papers, beginning in 1835 with a paper on the conditions of Vesuvius in 1834. His reports on Zoophytes, on the Geology of the Pacific Area, and on Crustacea, summarizing his work on the Wilkes Expedition, appeared from 1846 onward. Other works included Manual of Mineralogy (1848), afterwards entitled Manual of Mineralogy and Lithology (ed. 4, 1887); and Corals and Coral Islands] (1872; revised ed. 1890). In 1887, Dana revisited the Hawaiian Islands, and the results of his further investigations were published in a quarto volume entitled Characteristics of Volcanoes (1890).
The Manual of Mineralogy by J. D. Dana became a standard college text, and has been continuously revised and updated by a succession of editors including W. E. Ford (13th-14th eds., 1912–1929), Cornelius S. Hurlbut (15th-21st eds., 1941–1999), and beginning with the 22nd by Cornelis Klein. The 23rd edition is now in print under the title Manual of Mineral Science (Manual of Mineralogy) (2007), revised by Cornelis Klein and Barbara Dutrow.
Dana's System of Mineralogy has also been revised, the 6th edition (1892)[6] being edited by his son, #17 - Edward Salisbury Dana. A 7th edition was published in 1944, and the 8th edition was published in 1997 under the title Dana's New Mineralogy, edited by R. V. Gaines et al.
Between 1856 and 1857, Dana published a number of manuscripts in an effort to reconcile scientific findings with the Bible. Among these, he wrote Science and the Bible: A Review of the Six Days of Creation (1856), and Creation, Or, The Biblical Cosmogony in the Light of Modern Science (1885).

1869 - #12. Dmitri Ivanovich Mendeleev (1834 – 1907).
Mendeleev and Moseley are credited with being most responsible for the modern periodic law, which states that
when elements are arranged in order of increasing atomic number, there is a periodic repetition of their chemical and physical properties.
In his time, chemistry was a patchwork of guesses, observations and discoveries.
Mendeleev was certain that better, more fundamental principles could be found; this was his mindset when, in 1869, he began writing a second volume of his book The Principles of Chemistry.
At the heart of chemistry were its elements. What, wondered Mendeleev, could they reveal to him if he could find some way of organizing them logically?
He wrote the names of the 65 known elements on cards – much like playing cards – one element on each card. He then wrote the fundamental properties of every element on its own card, including atomic weight. He saw that atomic weight was important in some way – the behavior of the elements seemed to repeat as their atomic weights increased – but he could not see the pattern.
Convinced that he was close to discovering something significant, Mendeleev moved the cards about for hour after hour until finally he fell asleep at his desk.
When he awoke, he found that his subconscious mind had done his work for him! He now knew the pattern the elements followed. He later wrote:
“In a dream I saw a table where all the elements fell into place as required. Awakening, I immediately wrote it down on a piece of paper.”

Chart 3.
The result is the periodic table as we know it today (Figure below).
We now know that each new horizontal row of the periodic table corresponds to the beginning of a new principal energy level being filled with electrons. Elements with similar chemical properties appear at regular intervals, within the vertical columns called groups.

Chart 4.

Chart 5.


1875 - #13 George Jarvis Brush (1831–1912) was an American mineralogist born in Brooklyn, New York. He began his studies at Yale in 1848 with courses from Benjamin Silliman, Jr. and John Pitkin Norton on practical chemistry and agriculture. He also studied chemistry, metallurgy and mineralogy. From 1852 to 1855, Brush worked and studied at the University of Virginia and in Munich and Freiberg. He returned to Sheffield in 1855 to join the faculty as professor of Metallurgy and later of Mineralogy. Brush had begun acquiring an extensive research collection of minerals. He was appointed the first curator of the Peabody Museum of Natural History's mineral collection. He published extensively in the American Journal of Science and other journals.
He also published a Manual of Determinative Mineralogy (1875; fifteenth edition, 1899).


1912 - #14 Alfred Lothar Wegener (1 November 1880 – November 1930) suggested The theory of Continental drift, but the idea wasn’t accepted until circa 1955.

1913 - #15 Henry Gwyn Jeffreys Moseley (23 November 1887 – 10 August 1915)Perfected the Periodic table.
Probably would have become one of our most esteemed scientists had he not been killed at the tender age of 27 in the First World War by a Muslim sniper bullet.
In 1913 - Moseley observed and measured the X-ray spectra of various chemical elements (mostly metals) that were found by the method of diffraction through crystals. This was a pioneering use of the method of X-ray spectroscopy in physics, using Bragg's diffraction law to determine the X-ray wavelengths. Moseley discovered a systematic mathematical relationship between the wavelengths of the X-rays produced and the atomic numbers of the metals that were used as the targets in X-ray tubes. This has become known as Moseley's law.
Moseley's Law (spectroscopy)
The law that
the square-root of the frequency of an x-ray spectral line belonging to a particular series is proportional to the difference between the atomic number and a constant which depends only on the series.


Before Moseley's discovery, the atomic numbers (or elemental number) of an element had been thought of as a semi-arbitrary sequential number, based on the sequence of atomic masses, but modified somewhat where chemists found this modification to be desirable, such as by the Russian chemist, Dmitri Ivanovich Mendeleev.
In his invention of the Periodic Table of the Elements, Mendeleev had interchanged the orders of a few pairs of elements in order to put them in more appropriate places in this table of the elements. For example, the metals cobalt and nickel had been assigned the atomic numbers 27 and 28, respectively, based on their known chemical and physical properties, even though they have nearly the same atomic masses. In fact, the atomic mass of cobalt is slightly larger than that of nickel, which would have placed them in backwards order if they had been placed in the Periodic Table blindly according to atomic mass.
Moseley's experiments in X-ray spectroscopy showed directly from their physics that cobalt and nickel have the different atomic numbers, 27 and 28, and that they are placed in the Periodic Table correctly by Moseley's objective measurements of their atomic numbers. Hence, Moseley's discovery demonstrated that the atomic numbers of elements are not just rather arbitrary numbers based on chemistry and the intuition of chemists, but rather, they have a firm experimental basis from the physics of their X-ray spectra.
In addition, Moseley showed that there were gaps in the atomic number sequence at numbers 43, 61, 72, and 75. These spaces are now known, respectively, to be the places of the radioactive synthetic elements technetium and promethium, and also the last two quite rare naturally occurring stable elements hafnium (discovered 1923) and rhenium (discovered 1925). Nothing about these four elements was known of in Moseley's lifetime, not even their very existence. Based on the intuition of a very experienced chemist, Dmitri Mendeleev had predicted the existence of a missing element in the Periodic Table, which was later found to be filled by technetium, and Bohuslav Brauner had predicted the existence of another missing element in this Table, which was later found to be filled by promethium. Henry Moseley's experiments confirmed these predictions, by showing exactly what the missing atomic numbers were, 43 and 61. In addition, Moseley predicted the two more undiscovered elements, those with the atomic numbers 72 and 75, and gave very strong evidence that there were no other gaps in the Periodic Table between the elements aluminum (atomic number 13) and gold (atomic number 79).
This latter question about the possibility of more undiscovered ("missing") elements had been a standing problem among the chemists of the world, particularly given the existence of the large family of the lanthanide series of rare earth elements. Moseley was able to demonstrate that these lanthanide elements, i.e. lanthanum through lutetium, must have exactly 15 members – no more and no less. The number of elements in the lanthanides had been a question that was very far from being settled by the chemists of the early 20th Century. They could not yet produce pure samples of all the rare-earth elements, even in the form of their salts, and in some cases they were unable to distinguish between mixtures of two very similar (adjacent) rare-earth elements from the nearby pure metals in the Periodic Table. For example, there was a so-called "element" that was even given the chemical name of "didymium". "Didymium" was found some years later to be simply a mixture of two genuine rare-earth elements, and these were given the names neodymium and praseodymium, meaning "new twin" and "green twin". Also, the method of separating the rare-earth elements by the method of ion exchange had not been invented yet in Moseley's time.
Moseley's method in early X-ray spectroscopy was able to sort out the above chemical problems promptly, some of which had occupied chemists for a number of years. Moseley also predicted the existence of element 61, a lanthanide whose existence was previously unsuspected. Quite a few years later, this element 61 was created artificially in nuclear reactors and was named promethium.[12]
Isaac Asimov wrote,
"In view of what he [Moseley] might still have accomplished … his death might well have been the most costly single death of the War to mankind generally."
Isaac Asimov also speculated that, in the event that he had not been killed while in the service of the British Empire, Moseley might very well have been awarded the 1916 Nobel Prize in Physics, which, along with the prize for chemistry, was not awarded to anyone that year.

1928 - #16 Norman Levi Bowen was born in Kingston, Ontario, Canada June 21, 1887 and died on September 11, 1956. Bowen "revolutionized experimental petrology and our understanding of mineral crystallization". Beginning geology students are familiar with Bowen's reaction series depicting how different minerals crystallize under varying pressures and temperatures."[3]
Bowen conducted experimental research at the Geophysical Laboratory, Carnegie Institution for Science of Washington from 1912 to 1937. He published The Evolution of the Igneous Rocks in 1928. This book set the stage for a geochemical and geophysical foundation for the study of rocks and minerals. This book became the petrology handbook.






1944 - #17 Edward Salisbury Dana (November 16, 1849 – June 16, 1935), the son of #11 - James Dana, was an American mineralogist and physicist. He made important contributions to the study of minerals, especially in the field of crystallography.
E. S. Dana was born in New Haven, Connecticut, the son of the geologist and mineralogist James Dwight Dana.[1] He graduated from Yale College in 1870, where he had been a member of Scroll and Key, and then after two years with George J. Brush at the Sheffield Scientific School, spent another two years studying in Heidelberg and Vienna, specializing in crystal optics and crystallography. He then returned to Yale to take his M.A. and Ph.D. degrees. He was a member of the Connecticut Academy of Arts and Sciences.
He was appointed assistant professor of natural philosophy and astronomy at Yale in 1879 and then became professor of physics.[1][2] His research and publishing was mainly in the field of mineralogy.
Dana became an editor of the American Journal of Science in 1875 and continued to direct it until 1926. In 1884 was elected a member of the National Academy of Sciences. In 1885 he was made a trustee of the Peabody Museum of Yale.[2] He was an elected member of scientific societies in Austria, Mexico, Russia, England, Scotland, and across the United States.

From Amazon.com:
Most Helpful Customer Reviews
5.0 out of 5 stars Best Introduction to Mineralogy out there!!!
By Ed Shaw on February 24, 2009
For anyone interested in learning the basics of mineralogy, this is the place to start! Easy to read and understand. Great section on the Crystal Systems. Most mineral or mineralogy books spend most of the book on photographs, which is great, but this book focuses on giving the reader the basic understanding to know what they are looking at in the many fine "picture books" already out there about minerals.
5.0 out of 5 stars Dana's Minerals and How to Study Them
By Edwin Simmons on December 27, 2012
"Dana's Minerals and How to Study Them" came in the condition described by the seller. It is a classic work and an excellent addition to my library of books relating to the identification of minerals. It is an excellent balance between technical and practical.It is also a rare find. I would highly recommend it to other rock and mineral collectors.
4.0 out of 5 stars Not Your Average Rock Book
By Beth Amazon reviewer on April 20, 2013
This book is more of an erudite text that contains info you're not likely to get elsewhere. It's not your average rock and gem guide, so if you already own one or more of them but you're ready for something more in depth, this could be it.
5.0 out of 5 stars Best Introduction to Mineralogy out there!!!
By Ed Shaw on February 24, 2009
For anyone interested in learning the basics of mineralogy, this is the place to start! Easy to read and understand. Great section on the Crystal Systems. Most mineral or mineralogy books spend most of the book on photographs, which is great, but this book focuses on giving the reader the basic understanding to know what they are looking at in the many fine "picture books" already out there about minerals.
5.0 out of 5 stars Dana's Minerals and How to Study Them
By Edwin Simmons on December 27, 2012
"Dana's Minerals and How to Study Them" came in the condition described by the seller. It is a classic work and an excellent addition to my library of books relating to the identification of minerals. It is an excellent balance between technical and practical.It is also a rare find. I would highly recommend it to other rock and mineral collectors.
4.0 out of 5 stars Not Your Average Rock Book
By Beth Amazon reviewer on April 20, 2013
This book is more of an erudite text that contains info you're not likely to get elsewhere. It's not your average rock and gem guide, so if you already own one or more of them but you're ready for something more in depth, this could be it.