Post by 1dave on Nov 18, 2021 13:19:13 GMT -5
Here it is Son Of Beach, You asked for it.

As far as we know, the first person to wonder "how old is this rock?" was William Smith (1769–1834), an engineer and surveyor working in the Bath district of western England. He recognized that the earth was being laid down in layers like pages in a book, with the oldest pages at the bottom.
Radiocarbon dating (also referred to as carbon dating or carbon-14 dating) changed all that. It is a method for determining the age of an object containing organic material by using the properties of radiocarbon, a radioactive isotope of carbon.
No other element has played a larger role than carbon on our planet. Without carbon there would be NO LIFE!
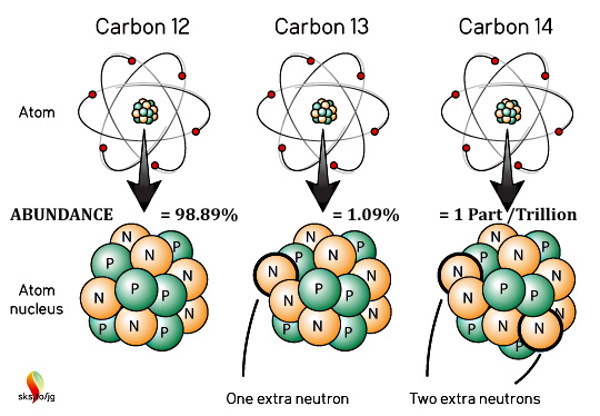
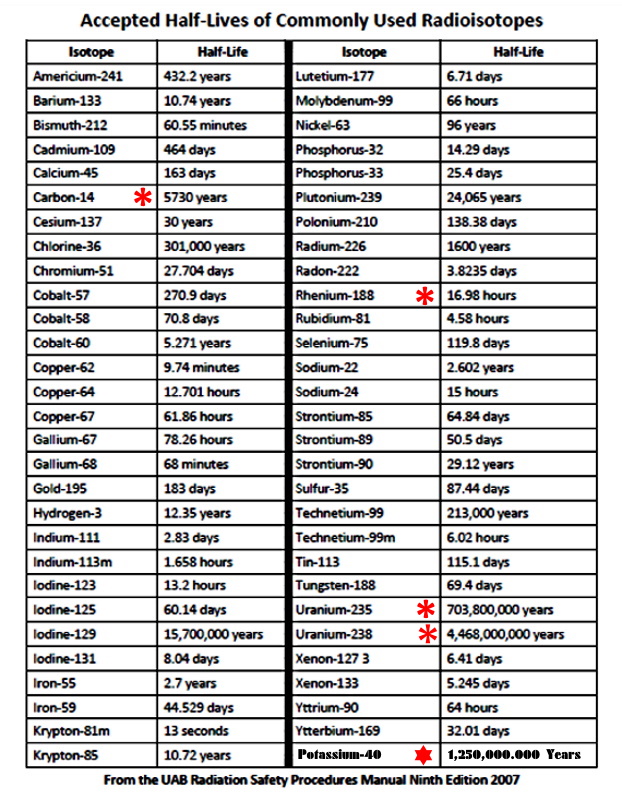
Carbon has as many as 15 isotopes (an uneven number of protons and neutrons), but most are ephemeral, only lasting from a few nanoseconds to a few minutes. Electrons, especially Valence Electrons come and go, but an imbalance of protons and neutrons is serious stuff. The three that are "naturally occurring" are 12C, 13C, and 14C.
12C is the normal carbon atom with 12 protons, 12 neutrons, and 12 electrons. BUT 13C has an extra neutron, and 14C has two!
Although we can't predict when an individual radioactive atom will decay, the rate at which a large collection of radioactive material decays is predictable and measured according to the isotope's half-life.
A half-life is the length of time it takes for half of the material to decay. Carbon-14 has a half-life of 5,730 years, so if you had a big block of carbon-14, in 5,730 years, half of the atoms in your block will have decayed back into the original nitrogen-14. After another 5,730 years, a total of 75% will have decayed, and after another 5,730 years, 87.5% will have decayed, and so on.
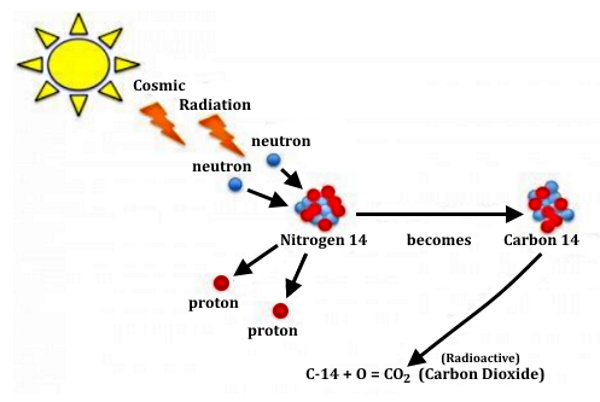
The Earth's air contains carbon dioxide, and some of the carbon in the CO₂ molecules is carbon-14. When plants take in carbon dioxide to grow, the carbon-14 becomes trapped in their cells, and anything that eats the plants, also eats the carbon-14. Because of this, radioactive carbon-14 is in every single organism on the planet, including you, but mercifully at such small levels it doesn't harm you; in fact, in some ways, it's extremely convenient.
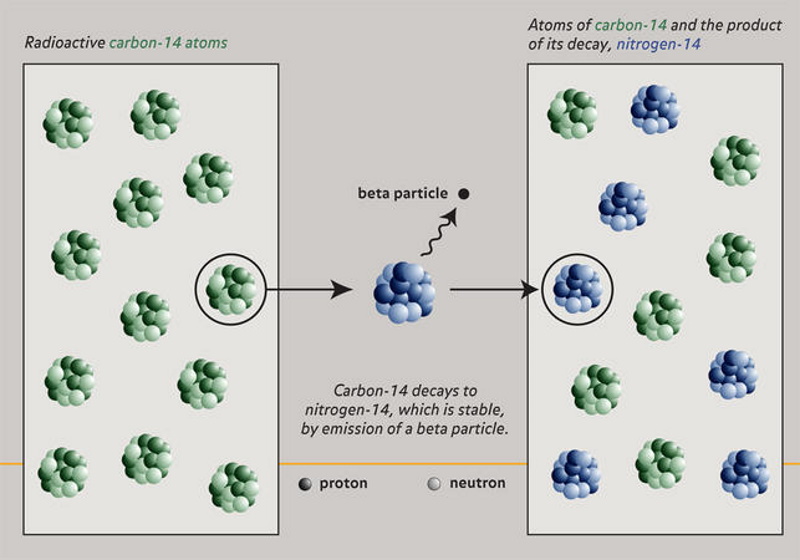
All living things consume carbon-14 their whole lives, but, when they die, no new carbon-14 enters the body, and the stockpile begins to slowly decay into nitrogen-14 which is trapped in solid parts of the organism. By using extremely sensitive equipment, scientists can determine the ratio between carbon-14 and nitrogen-14 in the remains and figure out how long ago the organism died. With a 75% carbon-14 to 25% nitrogen-14 ratio, we know about 2,850 years have passed, at 50%/50%, about 5,700 years have passed, and at 25%/75%, 11,400 years have passed, and so on. This is called radiocarbon dating, and you can use the same principle on numerous other radioactive elements, each with different half-lifes, a process called radiometric dating.
en.wikipedia.org/wiki/Radiocarbon_dating
The method was developed in the late 1940s at the University of Chicago by Willard Libby. It is based on the fact that radiocarbon (14C) is constantly being created in the Earth's atmosphere by the interaction of cosmic rays with atmospheric nitrogen. The resulting 14C combines with atmospheric oxygen to form radioactive carbon dioxide, which is incorporated into plants by photosynthesis; animals then acquire 14C by eating the plants. When the animal or plant dies, it stops exchanging carbon with its environment, and thereafter the amount of 14C it contains begins to decrease as the 14C undergoes radioactive decay. Measuring the amount of 14C in a sample from a dead plant or animal, such as a piece of wood or a fragment of bone, provides information that can be used to calculate when the animal or plant died. The older a sample is, the less 14C there is to be detected, and because the half-life of 14C (the period of time after which half of a given sample will have decayed) is about 5,730 years, the oldest dates that can be reliably measured by this process date to approximately 50,000 years ago, although special preparation methods occasionally make accurate analysis of older samples possible.
Libby received the Nobel Prize in Chemistry for his work in 1960.
Research has been ongoing since the 1960s to determine what the proportion of 14C in the atmosphere has been over the past fifty thousand years.
The resulting data, in the form of a calibration curve, is now used to convert a given measurement of radiocarbon in a sample into an estimate of the sample's calendar age. Other corrections must be made to account for the proportion of 14C in different types of organisms (fractionation), and the varying levels of 14C throughout the biosphere (reservoir effects). Additional complications come from the burning of fossil fuels such as coal and oil, and from the above-ground nuclear tests done in the 1950s and 1960s. Because the time it takes to convert biological materials to fossil fuels is substantially longer than the time it takes for its 14C to decay below detectable levels, fossil fuels contain almost no 14C. As a result, beginning in the late 19th century, there was a noticeable drop in the proportion of 14C as the carbon dioxide generated from burning fossil fuels began to accumulate in the atmosphere. Conversely, nuclear testing increased the amount of 14C in the atmosphere, which reached a maximum in about 1965 of almost double the amount present in the atmosphere prior to nuclear testing.
Measurement of radiocarbon was originally done by beta-counting devices, which counted the amount of beta radiation emitted by decaying 14C atoms in a sample. More recently, accelerator mass spectrometry has become the method of choice; it counts all the 14C atoms in the sample and not just the few that happen to decay during the measurements; it can therefore be used with much smaller samples (as small as individual plant seeds), and gives results much more quickly. The development of radiocarbon dating has had a profound impact on archaeology. In addition to permitting more accurate dating within archaeological sites than previous methods, it allows comparison of dates of events across great distances. Histories of archaeology often refer to its impact as the "radiocarbon revolution". Radiocarbon dating has allowed key transitions in prehistory to be dated, such as the end of the last ice age, and the beginning of the Neolithic and Bronze Age in different regions.
Now many more are available for cross-checking.

As far as we know, the first person to wonder "how old is this rock?" was William Smith (1769–1834), an engineer and surveyor working in the Bath district of western England. He recognized that the earth was being laid down in layers like pages in a book, with the oldest pages at the bottom.
Different methods of radiometric dating vary in the timescale over which they are accurate and the materials to which they can be applied.
Radiocarbon dating (also referred to as carbon dating or carbon-14 dating) changed all that. It is a method for determining the age of an object containing organic material by using the properties of radiocarbon, a radioactive isotope of carbon.

No other element has played a larger role than carbon on our planet. Without carbon there would be NO LIFE!
Carbon has as many as 15 isotopes (an uneven number of protons and neutrons), but most are ephemeral, only lasting from a few nanoseconds to a few minutes. Electrons, especially Valence Electrons come and go, but an imbalance of protons and neutrons is serious stuff. The three that are "naturally occurring" are 12C, 13C, and 14C.
12C is the normal carbon atom with 12 protons, 12 neutrons, and 12 electrons. BUT 13C has an extra neutron, and 14C has two!

Although we can't predict when an individual radioactive atom will decay, the rate at which a large collection of radioactive material decays is predictable and measured according to the isotope's half-life.
A half-life is the length of time it takes for half of the material to decay. Carbon-14 has a half-life of 5,730 years, so if you had a big block of carbon-14, in 5,730 years, half of the atoms in your block will have decayed back into the original nitrogen-14. After another 5,730 years, a total of 75% will have decayed, and after another 5,730 years, 87.5% will have decayed, and so on.

The Earth's air contains carbon dioxide, and some of the carbon in the CO₂ molecules is carbon-14. When plants take in carbon dioxide to grow, the carbon-14 becomes trapped in their cells, and anything that eats the plants, also eats the carbon-14. Because of this, radioactive carbon-14 is in every single organism on the planet, including you, but mercifully at such small levels it doesn't harm you; in fact, in some ways, it's extremely convenient.
All living things consume carbon-14 their whole lives, but, when they die, no new carbon-14 enters the body, and the stockpile begins to slowly decay into nitrogen-14 which is trapped in solid parts of the organism. By using extremely sensitive equipment, scientists can determine the ratio between carbon-14 and nitrogen-14 in the remains and figure out how long ago the organism died. With a 75% carbon-14 to 25% nitrogen-14 ratio, we know about 2,850 years have passed, at 50%/50%, about 5,700 years have passed, and at 25%/75%, 11,400 years have passed, and so on. This is called radiocarbon dating, and you can use the same principle on numerous other radioactive elements, each with different half-lifes, a process called radiometric dating.
en.wikipedia.org/wiki/Radiocarbon_dating
The method was developed in the late 1940s at the University of Chicago by Willard Libby. It is based on the fact that radiocarbon (14C) is constantly being created in the Earth's atmosphere by the interaction of cosmic rays with atmospheric nitrogen. The resulting 14C combines with atmospheric oxygen to form radioactive carbon dioxide, which is incorporated into plants by photosynthesis; animals then acquire 14C by eating the plants. When the animal or plant dies, it stops exchanging carbon with its environment, and thereafter the amount of 14C it contains begins to decrease as the 14C undergoes radioactive decay. Measuring the amount of 14C in a sample from a dead plant or animal, such as a piece of wood or a fragment of bone, provides information that can be used to calculate when the animal or plant died. The older a sample is, the less 14C there is to be detected, and because the half-life of 14C (the period of time after which half of a given sample will have decayed) is about 5,730 years, the oldest dates that can be reliably measured by this process date to approximately 50,000 years ago, although special preparation methods occasionally make accurate analysis of older samples possible.
Libby received the Nobel Prize in Chemistry for his work in 1960.
Research has been ongoing since the 1960s to determine what the proportion of 14C in the atmosphere has been over the past fifty thousand years.
The resulting data, in the form of a calibration curve, is now used to convert a given measurement of radiocarbon in a sample into an estimate of the sample's calendar age. Other corrections must be made to account for the proportion of 14C in different types of organisms (fractionation), and the varying levels of 14C throughout the biosphere (reservoir effects). Additional complications come from the burning of fossil fuels such as coal and oil, and from the above-ground nuclear tests done in the 1950s and 1960s. Because the time it takes to convert biological materials to fossil fuels is substantially longer than the time it takes for its 14C to decay below detectable levels, fossil fuels contain almost no 14C. As a result, beginning in the late 19th century, there was a noticeable drop in the proportion of 14C as the carbon dioxide generated from burning fossil fuels began to accumulate in the atmosphere. Conversely, nuclear testing increased the amount of 14C in the atmosphere, which reached a maximum in about 1965 of almost double the amount present in the atmosphere prior to nuclear testing.
Measurement of radiocarbon was originally done by beta-counting devices, which counted the amount of beta radiation emitted by decaying 14C atoms in a sample. More recently, accelerator mass spectrometry has become the method of choice; it counts all the 14C atoms in the sample and not just the few that happen to decay during the measurements; it can therefore be used with much smaller samples (as small as individual plant seeds), and gives results much more quickly. The development of radiocarbon dating has had a profound impact on archaeology. In addition to permitting more accurate dating within archaeological sites than previous methods, it allows comparison of dates of events across great distances. Histories of archaeology often refer to its impact as the "radiocarbon revolution". Radiocarbon dating has allowed key transitions in prehistory to be dated, such as the end of the last ice age, and the beginning of the Neolithic and Bronze Age in different regions.
Now many more are available for cross-checking.


 -
-














 Da Rules are up above in the Sales Department:
Da Rules are up above in the Sales Department:







 You're down here in the bilges with the wharf rats. You will get more mileage above on the upper decks.
You're down here in the bilges with the wharf rats. You will get more mileage above on the upper decks.

